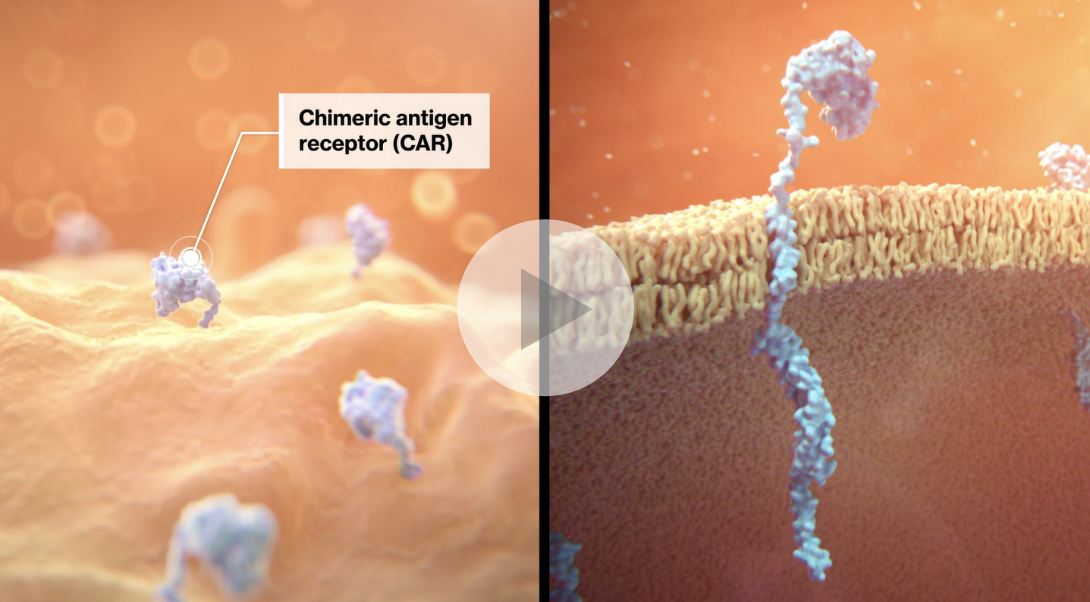
CAR-T cell therapy is a targeted, personalized therapy that involves the infusion of patients’ autologous T cells genetically reengineered to fight cancer.1
The T cells are altered to express a CAR, which combines the specificity of a monoclonal antibody with the cytotoxic and memory functions of T cells.2 CAR-T cells may multiply and differentiate into central or effector memory cells, which may promote the survival of CAR-T cells.3,4
After cell collection and cryopreservation, cell manufacturing, and infusion, these altered T cells can now fight and destroy the cancerous cells they could not find before.
Watch this video below to understand the mechanism of action for CAR-T cell therapy.
CAR-T cell therapy is a targeted, personalized therapy that involves the infusion of patients’ autologous T cells genetically reengineered to fight cancer.1
The T cells are altered to express a CAR, which combines the specificity of a monoclonal antibody with the cytotoxic and memory functions of T cells.2 CAR-T cells may multiply and differentiate into central or effector memory cells, which may promote the survival of CAR-T cells.3,4
After cell collection and cryopreservation, cell manufacturing, and infusion, these altered T cells can now fight and destroy the cancerous cells they could not find before.
Watch this video below to understand the mechanism of action for CAR-T cell therapy.
Blood from the patient is drawn and separated to collect their T cells through apheresis (leukapheresis). This process occurs over 3 to 6 hours. Within 24 hours, the leukapheresis material is cryopreserved and sent to a manufacturing facility for reprogramming.1
The patient’s cryopreserved cells are shipped via specialized courier to an FDA-approved manufacturing facility, where the patient’s cells are genetically reprogrammed into CAR-T cells.1
The patient may receive lymphodepleting chemotherapy to reduce the number of white blood cells and prepare the body for CAR-T cells. The patient receives their reprogrammed CAR-T cells during a single infusion, which can be administered in either an inpatient or outpatient setting at the hematologist’s discretion.1

Monitor the patient 2 to 3 times during the first week after infusion. The patient should stay close to their treatment center for at least 4 weeks to monitor and be treated for potential side effects. Routine long-term monitoring is recommended.1
After the infusion and the initial post-infusion monitoring period (at least 4 weeks), the patient may return to their medical team for ongoing monitoring and regular care appointments. Here are some materials to support patients with their follow-up care.
Before CAR-T cell therapy, pediatric and young adult patients with relapsed or refractory pALL or DLBCL had limited treatment options, low survival rates, and poorer outcomes. For many patients, treatments such as chemotherapy and bone marrow transplant are insufficient in achieving remission.
in response to treatment for B-cell ALL5,6,7
after experiencing refractory or relapsed pALL8
for frontline therapy diagnosed for DLBCL, the most common lymphoid cancer, and adults with refractory/ relapsed DLBCL9
with alternative therapies needed for these DLBCL patients10, and nearly 50% of the refractory/relapsed DLBCL patient population have no effective standard of care treatment options11
among all adult DLBCL patients who are eligible for transplant. Transplant also has a considerable risk of transplant related morbidity 12
following treatment (frontline chemoimmunotherapy) initiation20
a much more aggressive form of NHL; with a transformation risk of ~20% at 5 years and ~60% at 8 years of observation21
in second or later remission (24-month survival rate) 12
who were in their second or later remission (5-year survival rate) 13
However, medical breakthroughs and scientific advancements have improved the treatment landscape for pALL and DLBCL patients.
Timely patient identification and referrals to CAR-T experts is critical for better patient outcomes. Data shows T cell health deteriorates over time and is associated with lower response rates to CAR-T therapies.16 To minimize the impact on T cell health, monitor and consider your patients for this treatment option when their T cells are relatively healthy. Chemotherapy and additional treatments impact T cell health over time, therefore referrals for apheresis should also be considered prior to giving therapies that may compromise the leukapheresis product.17
Pediatric and young adult patients up to and including 25 years of age with pediatric B-cell acute lymphoblastic leukemia (pALL) that is refractory, in relapse post-transplant or in second or later relapse19, which includes those who:
Adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy19, which includes patients who are/have:
Note: Complete remission is not a requirement.
Adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy22, which includes patients who are/have:
Note: Complete remission is not a requirement.

CAR-T cell therapy is available at certified hospital sites around the globe and reimbursed in many markets. Patients seeking to receive treatment may be required to travel.
CAR-T therapy may be associated with certain risks such as cytokine release syndrome (CRS) and neurological toxicities, which may be severe or life-threatening. Please refer to the Prescribing Information for more details.

https://www.hcp.novartis.com/products/kymriah/diffuse-large-b-cell-lymphoma-adults/treatment-process/ (8/21 138566) – extracted from Novartis resource
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4861363/; Maus MV, Levine BL. Oncologist. 2016; 21:608-617
Kawalekar OU, O’Connor RS, Fraietta JA, et al. Immunity. 2016;44:380-390
Porter DL, Hwang WT, Frey NV, et al. Sci Transl Med. 2015;7:303ra139. doi:10.1126/scitranslmed.aac5415.
Eissa HM et al. Blood Cancer J. 2017;7(2):e531
Locatelli F et al. Blood. 2012;120(14):2807-2816.
Ko RH et al. J Clin Oncol. 2010;28(4):648-654.
Schrappe M et al. N Engl J Med. 2012;366(15):1371-1381.
Crump M et al. Blood 2017;130:1800–8
Harris LJ, Patel K, Martin M. Novel Therapies for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Int J Mol Sci. 2020;21(22):8553. Published 2020 Nov 13. doi:10.3390/ijms21228553
Karlovitch, S. (2021, October 12). Newer agents for DLBCL show potential for earlier use in the course of treatment. Targeted Oncology. Retrieved October 27, 2021, from https://www.targetedonc.com/view/newer-agents-for-dlbcl-show-potential-for-earlier-use-in-the-course-of-treatment.
Friedberg JW. Hematology Am Soc Hematol Educ Program. 2011;2011:498-505.
Bondarenko SN et al. CTT Journal. 2016;5(2):12-20.
New development in CAR-T cell therapy. (2017). PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5320663/
Efficacy and safety of second-generation CAR T-cell therapy in diffuse large B-cell lymphoma: A meta-analysis. (2020, October 1). PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7416618/
Das RK et al. Cancer Discov. 2019;9(4):492-499
Yakoub-Agha I, et al. Haematologica. 2020;105(2):297-316
Jain T, et al. Biol Blood Marrow Transplant. 2019;25(12):2305-2321
Kymriah [summary of product characteristics]. Nuremberg, Germany: Novartis Pharma GmbH; 2020.
Freedman A. Follicular lymphoma: 2015 update on diagnosis and management. Am J Hematol. 2015;90(12):1171-1178
Lossos, I. S., & Gascoyne, R. D. (2011, June). Transformation of follicular lymphoma. Best practice & research. Clinical haematology. Retrieved October 25, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3112479/
FDA approves Novartis Kymriah® Car-T cell therapy for adult patients with relapsed or refractory follicular lymphoma. Novartis. (2022, May 28). Retrieved October 20, 2022, from https://www.novartis.com/news/media-releases/fda-approves-novartis-kymriah-car-t-cell-therapy-adult-patients-relapsed-or-refractory-follicular-lymphoma